What Is the Solute in a Salt Solution Like Brine
The fourth step is a rapid consumption of the gold salt left in solution where the particle size increases rapidly from 52 to 77 nm with a decrease in the polydispersity to 10 observed from 50 to 70 min within solution. Brine utilisation for cooling and salt production in wind-driven seawater greenhouses.

Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
Salt retention by size exclusion will represent a paradigm shift for desalination membranes as solute and water transport in current TFC membranes is governed by a solutiondiffusion mechanism.

. A tank is field with 1000 liters of pure water. A subterranean brine associated with an oil field in Oklahoma yielded Arhodomonas aquaeolei an aerobic organic acid-metabolizing gram-negative motile rod growing in the range of 6 to 20 NaCl with an. Osmoprotectants can prevent the photosystem-salt interactions reducing ROS production.
If the solution gets dissolved then it is an unsaturated solution. If more solute does not dissolve in the given solution then it will be a saturated solution. Its physical state is generally the same as that of the solvent.
Applying a modernised controlled hydroponic system like NFT where the solution circulates crop yields can reach an average of 2. In extreme cases such as in bdelloid rotifers tardigrades brine shrimp. The solute can be present in any concentration in a solid-liquid solution.
Underground salt formations in the United States which were penetrated by meteoric waters which slowly solubilize the salt. Water with salinity above 50 ppt is brine water. It is necessary to determine the ion selectivity in a mixed salt feed solution because the interaction between the ion and the IEM is affected when other counter-ions are presented.
The driving force for this separation is an osmotic pressure gradient such that a draw solution of high concentration relative to that of the feed solution is used to induce a net flow of water through the. Osmosis occurs when a solute water passes from a low concentration to a high concentration of salt. Design and modelling.
If it was osmosis the water in the meat would move into the concentrated brine and dry the meat. Another brine solution contaning 008. Forward osmosis FO is an osmotic process that like reverse osmosis RO uses a semi-permeable membrane to effect separation of water from dissolved solutes.
With the increased salt concentration of brine the salt will accumulate much more quickly on the surface of absorbers as water evaporates leading to more severe fouling issues accelerated decrease of evaporation rate and the eventual collapse of devices. Understanding the saltwater separation mechanisms of reverse osmosis RO membranes is critical for the further development and optimization of RO technology. And η A and η B are the removal ratio of ion A and B in the ED process respectively.
For these reasons introduction of biosynthetic pathways which result in the creation of osmoprotectants in crops is a current area of research but inducing expression at significant amounts is currently posing a. It is sometimes heterogeneous. Used a static mixer in.
Its composition and the concentrations of its components is always fixed. Mix the salt solution of A and B ions. Brining is a diffusion process.
Choose the option below that is characteristic of a solution. This is considered to be an autocatalytic reduction on the surface of the nanoparticles. Brine containting 008 kg of salt per liter anters the tank at 5 liters per minute.
Add a few drops of solute like salt in the solution and try to stir by keeping the temperature constant. Its physical state is generally the same as that of the solvent. Article Download PDF View Record in Scopus Google Scholar.
If we heat and add solute to find it dissolved but excess dissolved salt forms precipitate then the solution is. Several strategies have been explored to maintain the high evaporation rate over a long period out of. Like skin and cell membranes from an area with a low concentration of solute to an area of.
Similar Acinetobacter-like bacteria. For the salt-lake brine with a high LMR a. A further study by Polte et al.
Desalination 426 2018 pp. The solution-diffusion SD model is widely used to describe water and salt transport in RO but it does not describe the intricate transport mechanisms of water molecules and ions through the membrane.

Dissolving And Back Again American Chemical Society
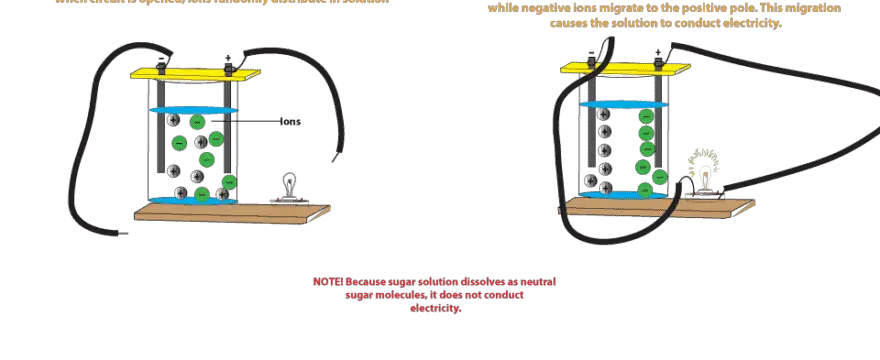
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
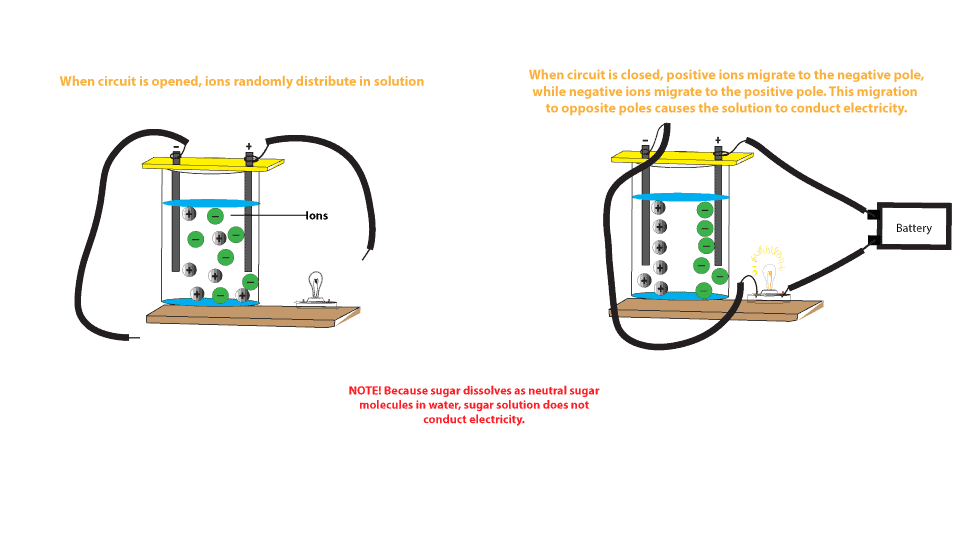
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
Comments
Post a Comment